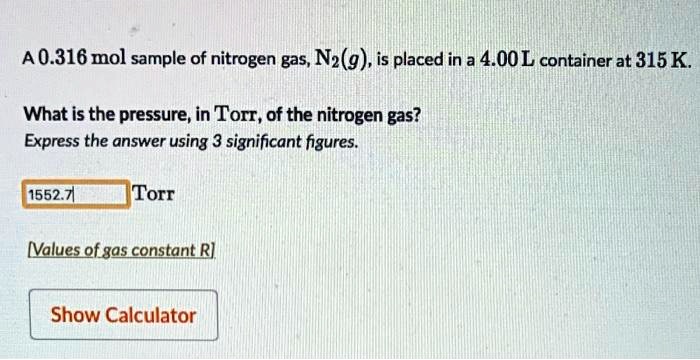
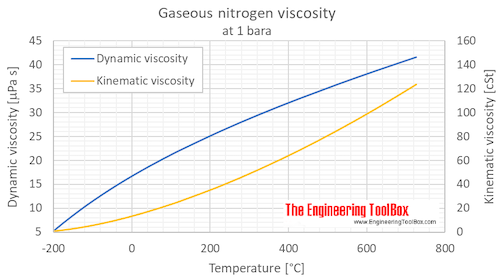
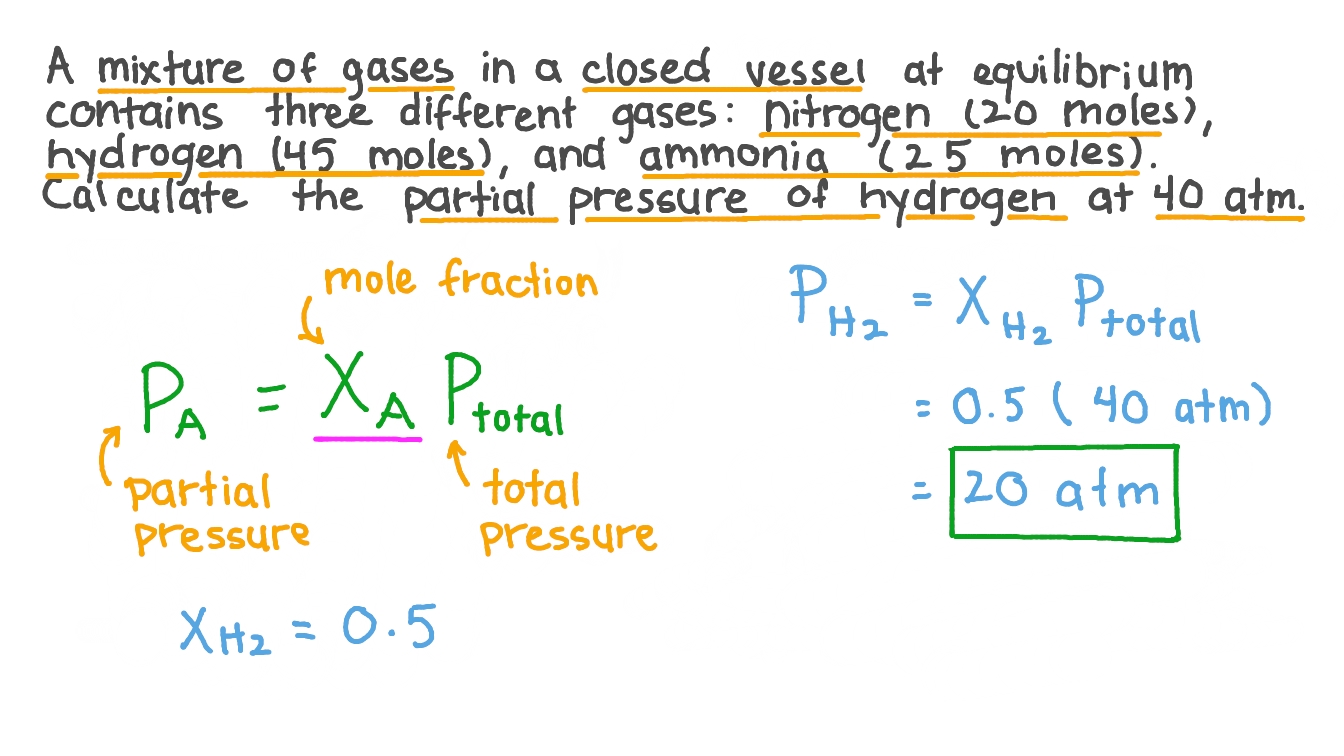
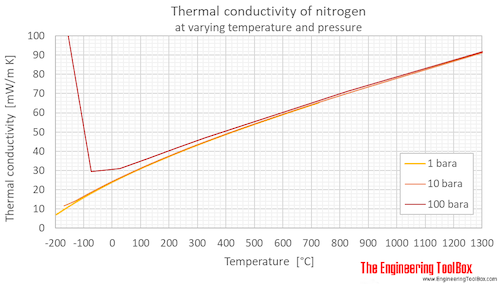
Calculate the mass of `3.011 xx 10^(24)` molecules of nitrogen gas `(N_(2))`. (Atomic mass : `N = 14
Packaged Nitrogen Gas / Containers / Items / [SCIM] Satisfactory - Calculator | Gaming Tool/Wiki/Database to empower the players.
Nitrogen Gas / Gas / Items / [SCIM] Satisfactory - Calculator | Gaming Tool/Wiki/Database to empower the players.

SOLVED: Calculate the mass of nitrogen gas (in mg) dissolved in a 6.00 L bucket of water exposed to a pressure of 1.47 atm of air. Assume the mole fraction of nitrogen