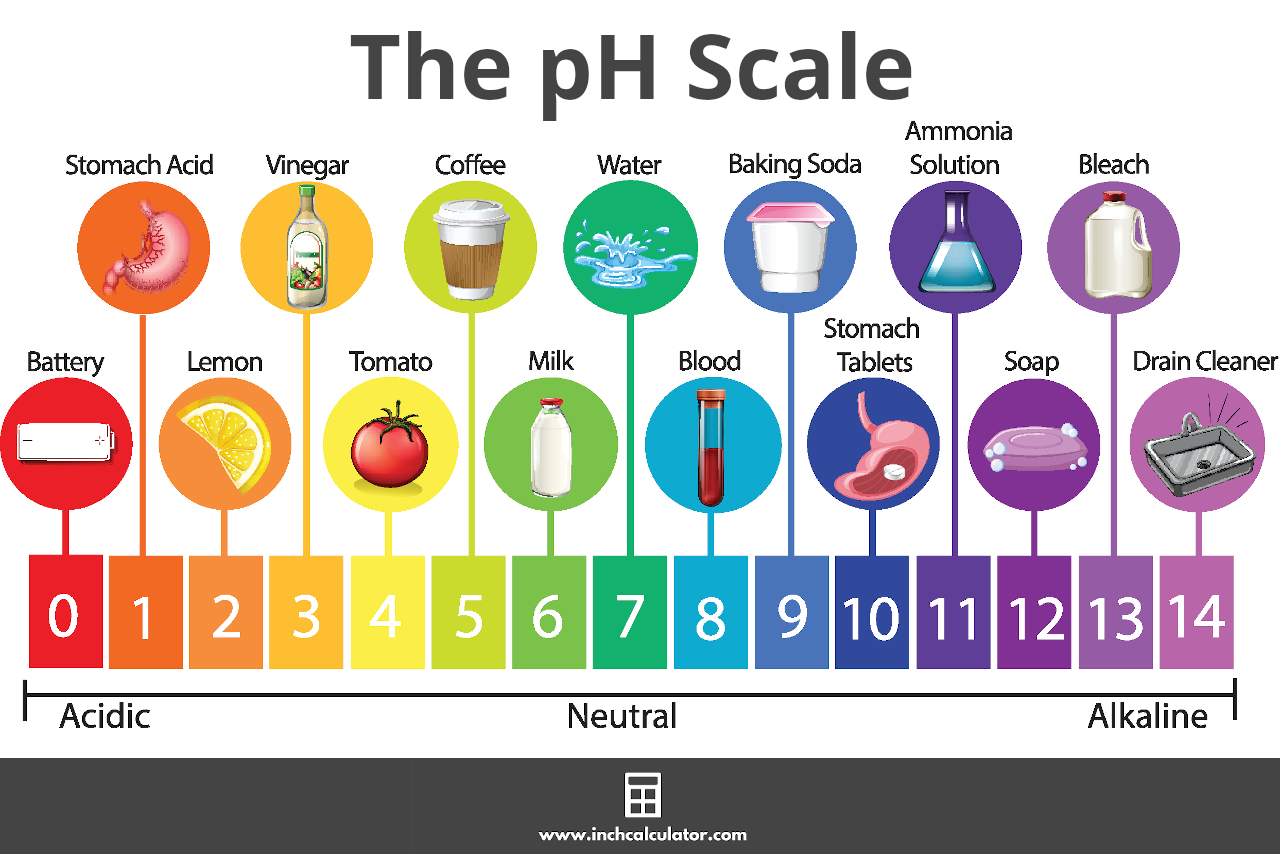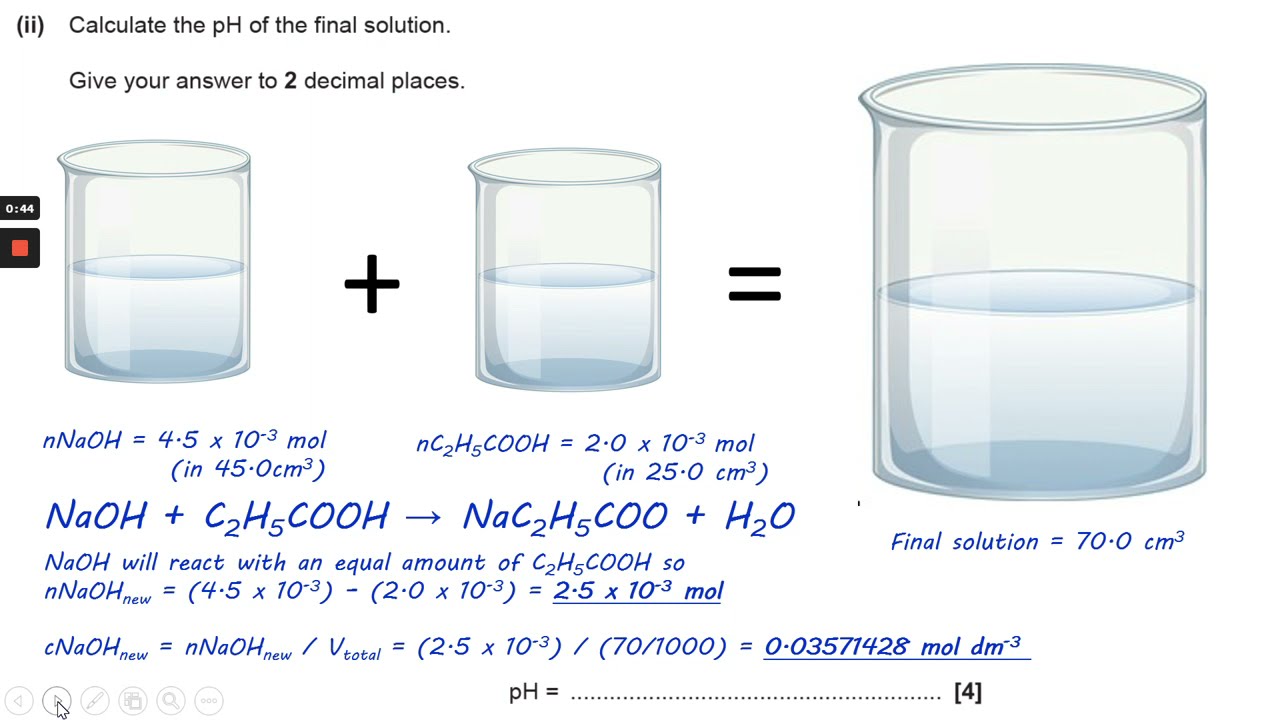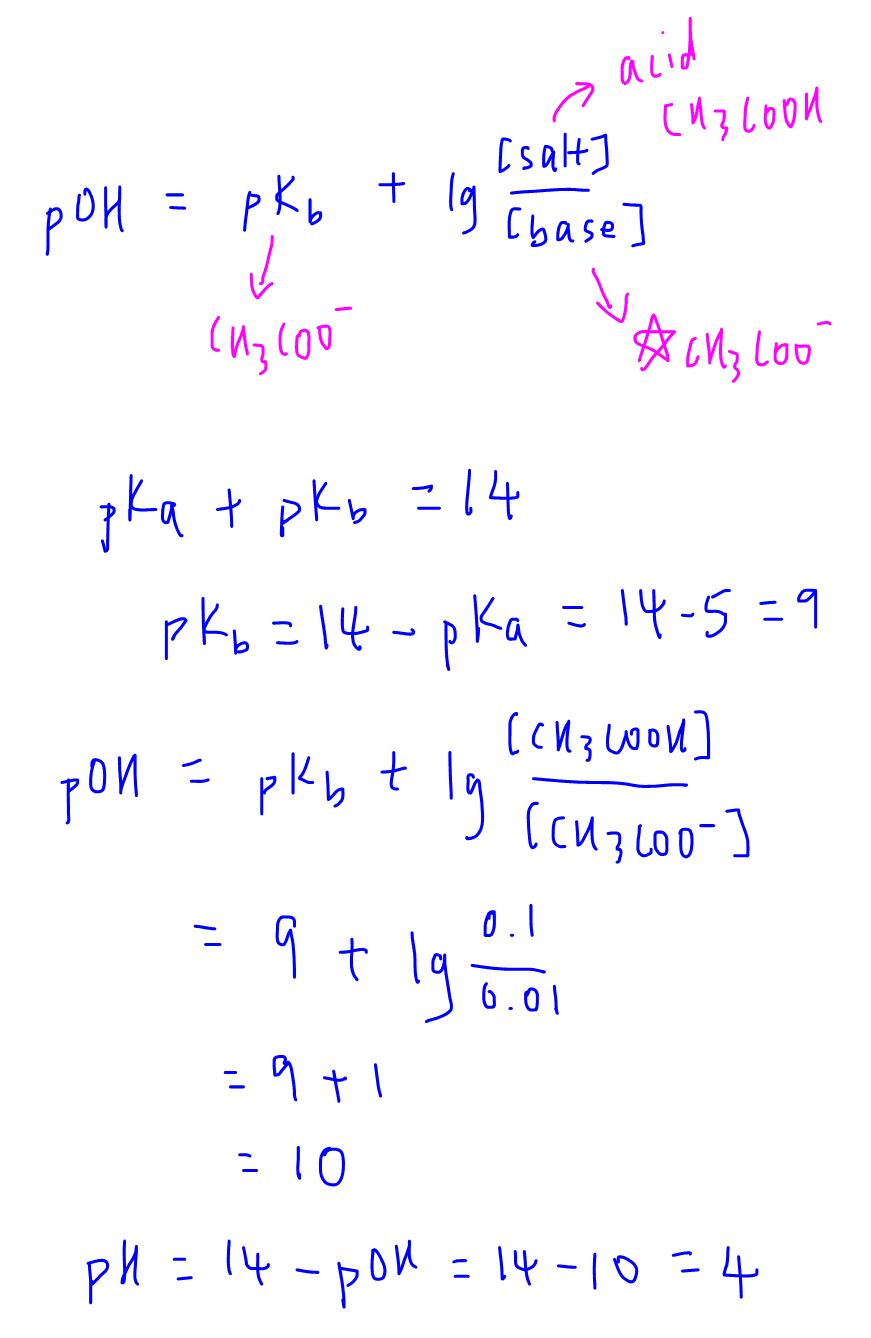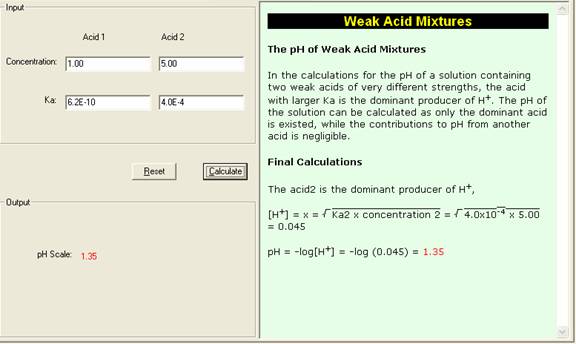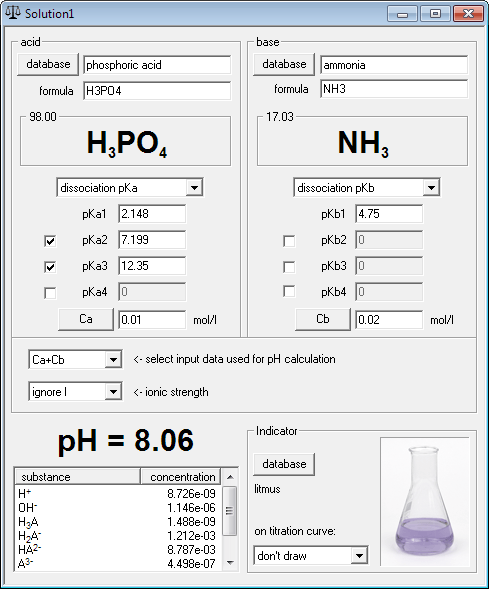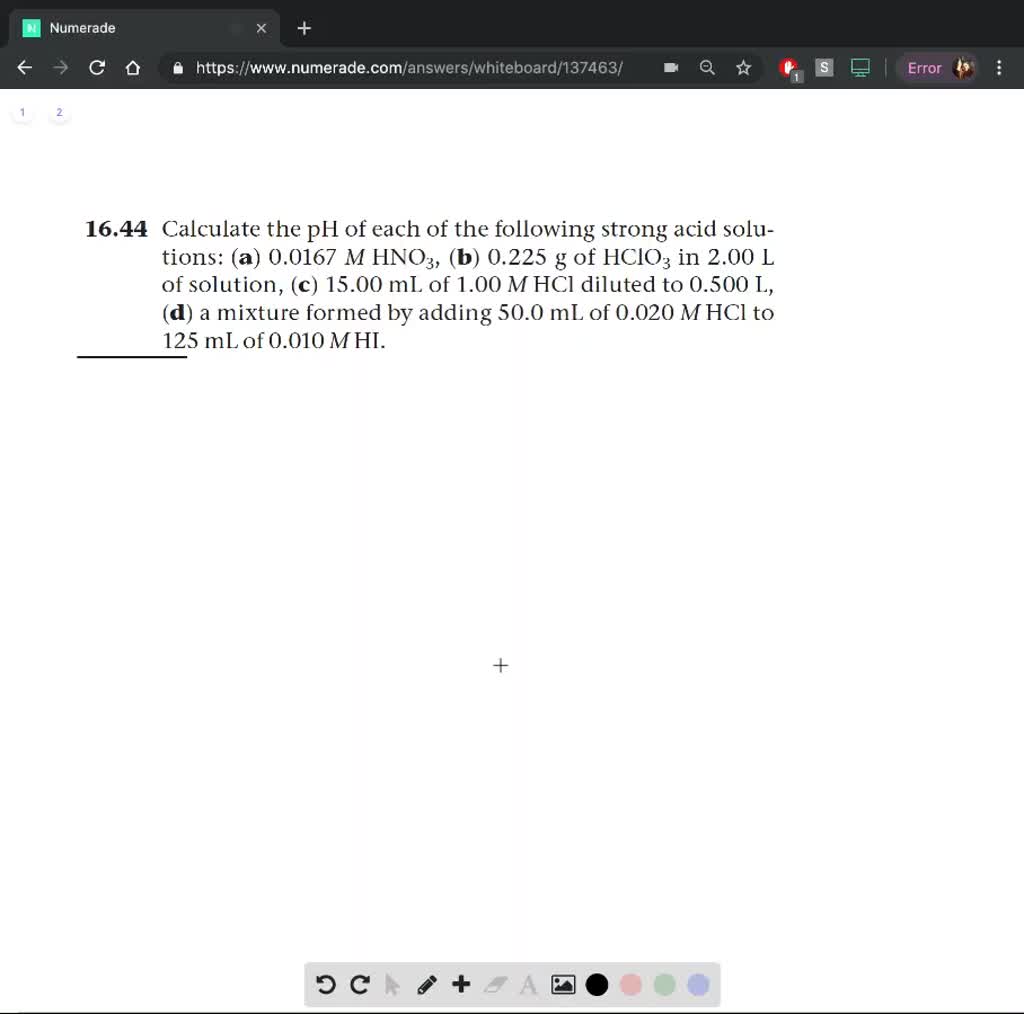
SOLVED: Calculate the pH of each of the following strong acid solutions: (a) 0.0167 M HNO3,(𝐛) 0.225 g of HClO3 in 2.00 L of solution, (𝐜) 15.00 mL of 1.00 M HCl
![PH calculations. What is pH? pH = - log 10 [H + (aq) ] where [H + ] is the concentration of hydrogen ions in mol dm -3 to convert pH into hydrogen ion. - ppt download PH calculations. What is pH? pH = - log 10 [H + (aq) ] where [H + ] is the concentration of hydrogen ions in mol dm -3 to convert pH into hydrogen ion. - ppt download](https://images.slideplayer.com/24/7441095/slides/slide_21.jpg)
PH calculations. What is pH? pH = - log 10 [H + (aq) ] where [H + ] is the concentration of hydrogen ions in mol dm -3 to convert pH into hydrogen ion. - ppt download
![PH calculations. What is pH? pH = - log 10 [H + (aq) ] where [H + ] is the concentration of hydrogen ions in mol dm -3 to convert pH into hydrogen ion. - ppt download PH calculations. What is pH? pH = - log 10 [H + (aq) ] where [H + ] is the concentration of hydrogen ions in mol dm -3 to convert pH into hydrogen ion. - ppt download](https://images.slideplayer.com/24/7441095/slides/slide_20.jpg)
PH calculations. What is pH? pH = - log 10 [H + (aq) ] where [H + ] is the concentration of hydrogen ions in mol dm -3 to convert pH into hydrogen ion. - ppt download
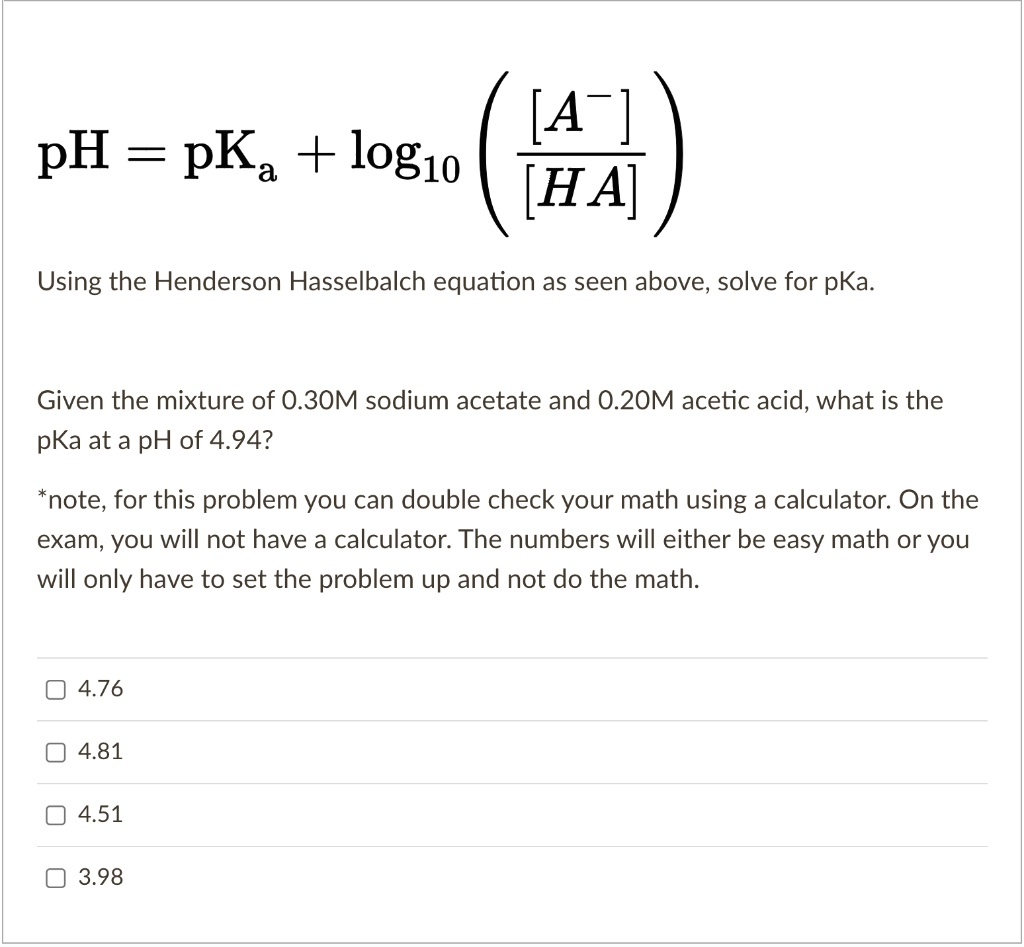
SOLVED: pH = pKa + log10 Using the Henderson-Hasselbalch equation as seen above, solve for pKa. Given the mixture of 0.30 M sodium acetate and 0.20 M acetic acid, what is the






:max_bytes(150000):strip_icc()/how-to-calculate-ph-quick-review-606089_final-165915b0177b4f6e82843f25097f51df.png)